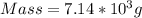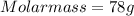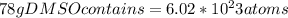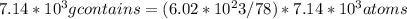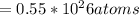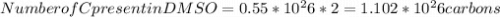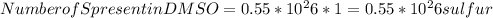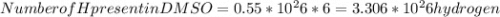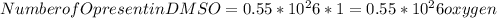Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2


Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Dimethyl sulfoxide [(ch3)2so], also called dmso, is an important solvent that penetrates the skin, e...
Questions

Mathematics, 27.11.2021 01:00






Biology, 27.11.2021 01:00

Business, 27.11.2021 01:00


Health, 27.11.2021 01:00


Business, 27.11.2021 01:00

Mathematics, 27.11.2021 01:00


Mathematics, 27.11.2021 01:00


History, 27.11.2021 01:00



![[(CH_3)_2 (SO) ]](/tpl/images/0077/5989/34170.png)