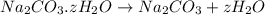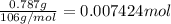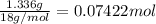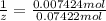Chemistry, 11.07.2019 19:00 ynclankaedon
Washing soda is a hydrated compound whose formula can be written na2co3 i zh2o, where z is the number of moles of h2o per mole of na2co3. when a 2.123 g sample of washing soda was heated at 130°c, all of the water of hydration was lost, leaving 0.787 g of anhydrous sodium carbonate. calculate the value of z.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 15:00
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3

Chemistry, 23.06.2019 15:00
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1
You know the right answer?
Washing soda is a hydrated compound whose formula can be written na2co3 i zh2o, where z is the numbe...
Questions


Mathematics, 08.07.2021 16:30

Computers and Technology, 08.07.2021 16:30

Mathematics, 08.07.2021 16:30





Computers and Technology, 08.07.2021 16:40

Mathematics, 08.07.2021 16:40

Computers and Technology, 08.07.2021 16:40

English, 08.07.2021 16:40

Physics, 08.07.2021 16:40



Mathematics, 08.07.2021 16:40


Computers and Technology, 08.07.2021 16:40


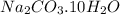 .
.