

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
A0.99 m aqueous solution of an ionic compound with the formula mx has a freezing point of -2.6 ∘c ....
Questions

Mathematics, 20.09.2021 14:10

Mathematics, 20.09.2021 14:10

Mathematics, 20.09.2021 14:10

Mathematics, 20.09.2021 14:10


Mathematics, 20.09.2021 14:10




History, 20.09.2021 14:10

Mathematics, 20.09.2021 14:10



Mathematics, 20.09.2021 14:10


History, 20.09.2021 14:10


Mathematics, 20.09.2021 14:10

Social Studies, 20.09.2021 14:10

Biology, 20.09.2021 14:10

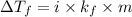 -(1)
-(1)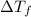 is depression in freezing point,
is depression in freezing point, is Van't Hoff factor,
is Van't Hoff factor,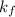 is molal freezing point depression constant, and
is molal freezing point depression constant, and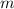 is molality of the solution.
is molality of the solution.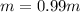 (given)
(given)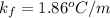
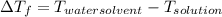
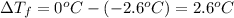
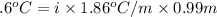
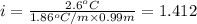
 is 1.412.
is 1.412.

