
Chemistry, 11.07.2019 19:00 imalexiscv
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.200 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq). what is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.200 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq). 0.167 m 0.333 m 0.120 m 0.0833 m?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions

Physics, 17.02.2021 17:30

History, 17.02.2021 17:30





Mathematics, 17.02.2021 17:30

English, 17.02.2021 17:30



Medicine, 17.02.2021 17:30

Mathematics, 17.02.2021 17:30

History, 17.02.2021 17:30


Mathematics, 17.02.2021 17:30

English, 17.02.2021 17:30

Biology, 17.02.2021 17:30

English, 17.02.2021 17:30

Social Studies, 17.02.2021 17:30

Mathematics, 17.02.2021 17:30

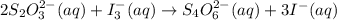
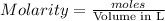
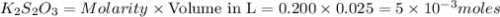
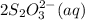 require 1 mole of
require 1 mole of 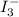
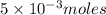 require=
require= moles of
moles of 


