
Chemistry, 11.07.2019 21:00 vjacksongonzalez
Naturally occurring neon exists as three isotopes. 90.51% is ne-20 with a mass of 19.99 amu, 0.27% is ne-21 with a mass of 20.99 amu, and 9.22% is ne-22 with a mass of 21.99 amu. what is the atomic mass of neon?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Naturally occurring neon exists as three isotopes. 90.51% is ne-20 with a mass of 19.99 amu, 0.27% i...
Questions


Mathematics, 12.03.2021 02:30




Mathematics, 12.03.2021 02:30

English, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30


English, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30


Mathematics, 12.03.2021 02:30




Mathematics, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30

 = ∑(
= ∑(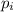 X
X 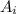 )/100. Where,
)/100. Where, 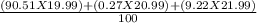 = 20.177 amu.
= 20.177 amu.

