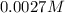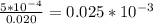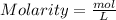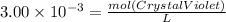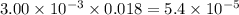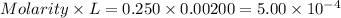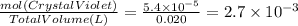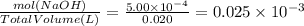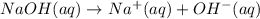Chemistry, 11.07.2019 21:30 aprilstalder
Asample was prepared by mixing 18. ml of 3.00 x 10^-3 m crystal violet (cv) with 2.00 ml of 0.250 m naoh. calculate the resulting concerntattions of cv and oh-

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Asample was prepared by mixing 18. ml of 3.00 x 10^-3 m crystal violet (cv) with 2.00 ml of 0.250 m...
Questions

Social Studies, 21.06.2019 13:30

English, 21.06.2019 13:30


Mathematics, 21.06.2019 13:30



Computers and Technology, 21.06.2019 13:30


History, 21.06.2019 13:30









Mathematics, 21.06.2019 13:30


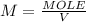
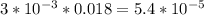
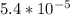
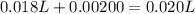
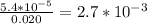 .
.