
Chemistry, 11.07.2019 23:30 steffweikeloyrt00
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of coffee with a temperature of 88.3 degrees c. assume the specific heats of coffee and milk are the same as for water (c = 4.19 j/g•c), and disregard the heat capacity of the container.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of co...
Questions


English, 15.07.2019 19:10

Mathematics, 15.07.2019 19:10

Mathematics, 15.07.2019 19:10


Mathematics, 15.07.2019 19:10





Mathematics, 15.07.2019 19:10


Computers and Technology, 15.07.2019 19:10




Computers and Technology, 15.07.2019 19:10



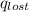 =
=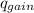 ,
, 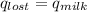 +
+ 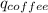
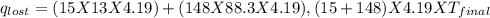 =(15X13X4.19)+(148X88.3X4.19)
=(15X13X4.19)+(148X88.3X4.19)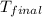 = 81.37 ° C
= 81.37 ° C

