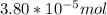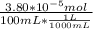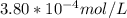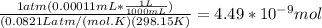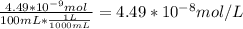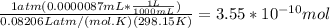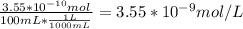A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in find how many moles of he are contained in 5.24 μl at 25.00°c (298.15 k) and 1.000 atm. this number is the molarity of he in the air. (2.11x10-7m) (b) find the molar concentrations of ar, kr, and xe in air at 25°c and 1 atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1


You know the right answer?
A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in f...
Questions

History, 20.11.2020 03:10

Mathematics, 20.11.2020 03:10

Mathematics, 20.11.2020 03:10

Biology, 20.11.2020 03:10

English, 20.11.2020 03:10



Mathematics, 20.11.2020 03:10

Mathematics, 20.11.2020 03:10

History, 20.11.2020 03:10




Mathematics, 20.11.2020 03:10

Mathematics, 20.11.2020 03:10

Mathematics, 20.11.2020 03:10



Mathematics, 20.11.2020 03:10

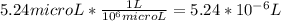
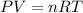
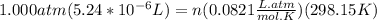
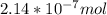
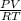
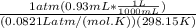 =
=