
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced equation: (ii)what would be observed if the solution was titrated well past the equivalence point using bromthymolblue as the indicator? (bromthymol blue is yellow in acidic solution and blue in basic solution.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced...
Questions



Health, 11.12.2019 15:31

Geography, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31




English, 11.12.2019 15:31




Mathematics, 11.12.2019 15:31


Physics, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31



Mathematics, 11.12.2019 15:31

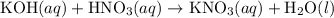
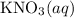 (and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result,
(and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result, 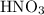 is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and
is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and 

