
Chemistry, 12.07.2019 02:00 rubianny03
Five mineral samples of equal mass of calcite, caco3 (mm 100.085) , had a total mass of 10.9 ± 0.1 g. what is the average mass of calcium in each sample? (assume that the relative uncertainties in atomic mass are small compared the uncertainty of the total mass.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Five mineral samples of equal mass of calcite, caco3 (mm 100.085) , had a total mass of 10.9 ± 0.1 g...
Questions



Spanish, 16.11.2020 04:00

Computers and Technology, 16.11.2020 04:00

Chemistry, 16.11.2020 04:00



Mathematics, 16.11.2020 04:00

Mathematics, 16.11.2020 04:00


Mathematics, 16.11.2020 04:00

English, 16.11.2020 04:00

History, 16.11.2020 04:00

Mathematics, 16.11.2020 04:00



Mathematics, 16.11.2020 04:00


History, 16.11.2020 04:00

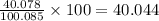 %
%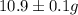 (given)
(given)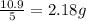
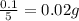

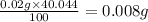
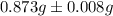 .
.

