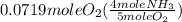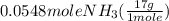Chemistry, 12.07.2019 02:30 Thejollyhellhound20
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of nh3 to no. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) in a certain experiment, 1.90 g of nh3 reacts with 2.30 g of o2. (a) which is the limiting reactant? o2 nh3 (b) how many grams of no and of h2o form? 1.73 g no 1.55 g h2o (c) how many grams of the excess reactant remain after the limiting reactant is completely consumed? g (d) show that your calculations in parts (b) and (c) are consistent with the law of conservation of mass. mass of products + excess reactant g total mass of reactants g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion o...
Questions



Social Studies, 06.05.2020 05:17


Mathematics, 06.05.2020 05:17


Biology, 06.05.2020 05:17


English, 06.05.2020 05:17

History, 06.05.2020 05:17


Mathematics, 06.05.2020 05:17

Mathematics, 06.05.2020 05:17

Biology, 06.05.2020 05:17






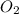 , (b) 1.73 g NO and 1.55 g
, (b) 1.73 g NO and 1.55 g 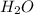 , (c) 0.932 g of
, (c) 0.932 g of 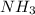 , (d) yes, the results are consistent with law of conservation of mass.
, (d) yes, the results are consistent with law of conservation of mass.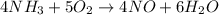
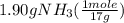 = 0.112 mole
= 0.112 mole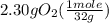 = 0.0719 mole
= 0.0719 mole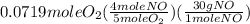 = 1.73 g of NO
= 1.73 g of NO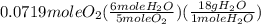 = 1.55 g of
= 1.55 g of