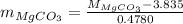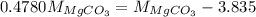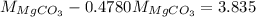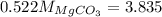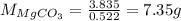Chemistry, 12.07.2019 02:30 dolahghazali76
A20.7167 g sample of impure magnesium carbonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g). after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 16.8817 g. assuming that only the magnesium carbonate had decomposed, how much magnesium carbonate was present in the original sample? answer in units of g. 013 5.0 points the reaction of 8.9 grams of fluo

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
A20.7167 g sample of impure magnesium carbonate was heated to complete decomposition according to th...
Questions

Mathematics, 05.07.2019 08:50

Mathematics, 05.07.2019 08:50

Physics, 05.07.2019 08:50



Mathematics, 05.07.2019 08:50





Mathematics, 05.07.2019 08:50

Mathematics, 05.07.2019 08:50







Mathematics, 05.07.2019 08:50

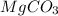 is 20.7167 g. The decomposition reaction is as follows:
is 20.7167 g. The decomposition reaction is as follows: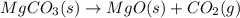
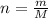
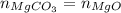
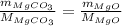
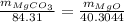
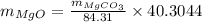
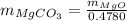 ...... (1)
...... (1)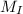 and mass of impure
and mass of impure 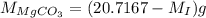 ...... (2)
...... (2)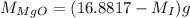 ...... (3)
...... (3)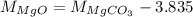
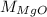 in equation (1),
in equation (1),