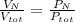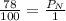Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25.0 ∘c, the n2 component will dissolve in water with a solubility of 4.88×10−4 m. what is the value of henry's law constant for n2 under these conditions? express your answer with the appropriate units. enter the unit m using the compound form mol/l.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25....
Questions



Social Studies, 09.12.2020 19:10

History, 09.12.2020 19:10

Spanish, 09.12.2020 19:10




Advanced Placement (AP), 09.12.2020 19:10



Biology, 09.12.2020 19:10





Computers and Technology, 09.12.2020 19:10



Physics, 09.12.2020 19:10