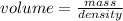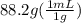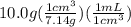Chemistry, 12.07.2019 08:30 nickboy52210
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10.0g of zinc (d=7.14 g/cm^3) are added to the flask filled with water (and the sides of the flask are dried from the displaced water) what is the new volume in the flask? (hint: you are going to have to use the density of water to answer this question)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10...
Questions



Mathematics, 17.11.2021 14:00



Computers and Technology, 17.11.2021 14:00

History, 17.11.2021 14:00

Law, 17.11.2021 14:00



Mathematics, 17.11.2021 14:00





Geography, 17.11.2021 14:00

Chemistry, 17.11.2021 14:30

Mathematics, 17.11.2021 14:30


Biology, 17.11.2021 15:10

 .
.