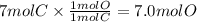Chemistry, 12.07.2019 09:00 rajene9302
This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel refining): nico4a chemical engineer has determined by measurements that there are 7.0 moles of carbon in a sample of nickel tetracarbonyl. how many moles of oxygen are in the sample? round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel...
Questions


Mathematics, 13.10.2020 03:01




Social Studies, 13.10.2020 03:01





Social Studies, 13.10.2020 03:01


Mathematics, 13.10.2020 03:01


Mathematics, 13.10.2020 03:01