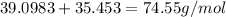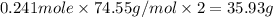Chemistry, 12.07.2019 09:00 mcdonaldmacy01
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calculate the elemental percent composition by mass of cisplatin. b. cisplatin is synthesized as follows: k2ptcl4 (aq) + 2nh3(aq) â pt(nh3)2cl2 (s) + 2kcl (aq) what mass of cisplatin can be made from 100.g of k2ptcl4 and sufficient nh3? what mass of kcl is also produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calcula...
Questions

History, 29.10.2020 05:20



Geography, 29.10.2020 05:20

Mathematics, 29.10.2020 05:20


Biology, 29.10.2020 05:20

Health, 29.10.2020 05:20

Physics, 29.10.2020 05:20



Social Studies, 29.10.2020 05:20

History, 29.10.2020 05:20

Physics, 29.10.2020 05:20


Physics, 29.10.2020 05:20

Mathematics, 29.10.2020 05:20



History, 29.10.2020 05:20

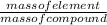 (1)
(1)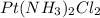 .
.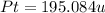
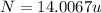
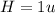
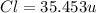
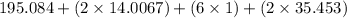 =
= 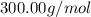
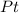 %
%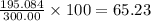 %.
%.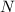 %
%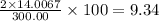 %
%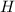 %
%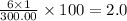 %
%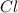 %
%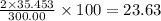 %
%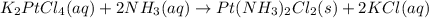
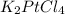 gives 1 mole of
gives 1 mole of 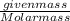
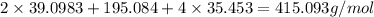
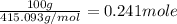 .
.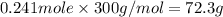
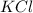 :
: