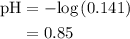Chemistry, 12.07.2019 11:30 brisamauro27
A50.0-ml sample of 0.50 m hcl is titrated with 0.50 m naoh. what is the ph of the solution after 28.0 ml of naoh have been added to the acid? 14) a.2.85 b.0.85 c.2.96 d.3.81 e.1.49

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
A50.0-ml sample of 0.50 m hcl is titrated with 0.50 m naoh. what is the ph of the solution after 28....
Questions

Mathematics, 26.02.2021 22:30

Mathematics, 26.02.2021 22:30





Mathematics, 26.02.2021 22:30

History, 26.02.2021 22:30

Mathematics, 26.02.2021 22:30




Mathematics, 26.02.2021 22:30


Mathematics, 26.02.2021 22:30





Mathematics, 26.02.2021 22:30

 .
.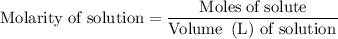
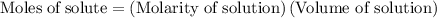 ......(2)
......(2)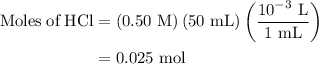
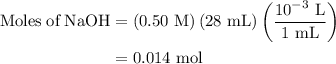
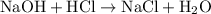
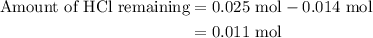
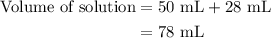
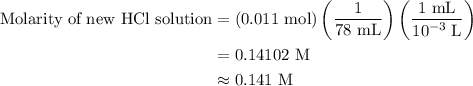
![{\text{pH}}=- {\text{log}}\left[ {{{\text{H}}^ + }}\right]](/tpl/images/0080/7989/2a671.png) ......(3)
......(3)![\left[{{{\text{H}}^ + }}\right]](/tpl/images/0080/7989/0bedd.png) is hydrogen ion concentration.
is hydrogen ion concentration.