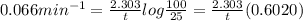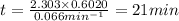Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
In a first-order decomposition reaction, 50.0% of a compound decomposes in 10.5 min. a. what is the...
Questions

Mathematics, 21.05.2020 12:58


Social Studies, 21.05.2020 12:58

Mathematics, 21.05.2020 12:58

Mathematics, 21.05.2020 12:58

Mathematics, 21.05.2020 12:58

Mathematics, 21.05.2020 12:58

Physics, 21.05.2020 12:58

History, 21.05.2020 12:58


Mathematics, 21.05.2020 12:58


Mathematics, 21.05.2020 12:58

Mathematics, 21.05.2020 12:58


Mathematics, 21.05.2020 12:58

History, 21.05.2020 12:59

Mathematics, 21.05.2020 12:59

History, 21.05.2020 12:59

Mathematics, 21.05.2020 12:59

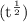
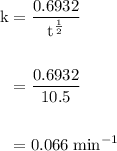
![\rm k = \dfrac{2.303}{t^{\frac{1}{2}}}log \dfrac{[A_{o}]}{[A_{t}]}](/tpl/images/0080/8236/dc3b5.png)
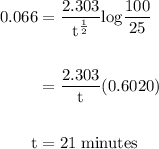
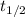 .
.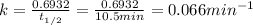
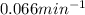 .
.![k=\frac{2.303}{t_{1/2}}log\frac{[A_{0}]}{[A_{t}]}](/tpl/images/0080/8236/f566d.png)
![[A_{0} ]](/tpl/images/0080/8236/f5a28.png) is 100 then concentration at time t
is 100 then concentration at time t ![[A_{t} ]](/tpl/images/0080/8236/7aeb5.png) will be 100-75=25.
will be 100-75=25.