
Chemistry, 12.07.2019 12:00 jholland03
Mercury (a. k.a. quicksilver) is a metallic element and a liquid at room temperature. calculate mercury\'s density if a sample of mercury is found to have a mass of 589.0 g and a volume of 43.86 ml.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Mercury (a. k.a. quicksilver) is a metallic element and a liquid at room temperature. calculate merc...
Questions



Mathematics, 07.12.2020 18:00




Arts, 07.12.2020 18:00

English, 07.12.2020 18:00










World Languages, 07.12.2020 18:00

Mathematics, 07.12.2020 18:00

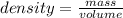 -(1)
-(1)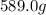 (given)
(given) (given)
(given)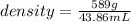
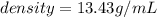
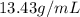 .
.


