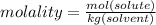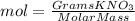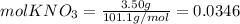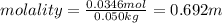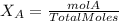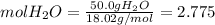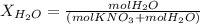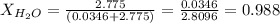Chemistry, 12.07.2019 12:00 Pizzapegasus1
Calculate the molality and mole fraction of water, respectively, of a solution that is made by dissolving 3.50 g of potassium nitrate in 50.0 g of water. the final volume of the solution is 56.0 ml.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Calculate the molality and mole fraction of water, respectively, of a solution that is made by disso...
Questions

Mathematics, 28.11.2021 22:00




Physics, 28.11.2021 22:00

English, 28.11.2021 22:00

History, 28.11.2021 22:00




English, 28.11.2021 22:00



Mathematics, 28.11.2021 22:00


Mathematics, 28.11.2021 22:00


Biology, 28.11.2021 22:00