
Chemistry, 12.07.2019 12:00 burnsmykala23
Which atom in the pictured molecule will have the strongest partial positive charge? there is a ball-and-stick model of nh2chrco2h in which the first oxygen is attached to carbon by double bond and another oxygen is attached by single bond. which atom in the pictured molecule will have the strongest partial positive charge? there is a ball-and-stick model of nh2chrco2h in which the first oxygen is attached to carbon by double bond and another oxygen is attached by single bond. the h that's bound to o. the n atom. the c that's in c=o. the c that's bound to n. the o atom that's in c=o?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Which atom in the pictured molecule will have the strongest partial positive charge? there is a bal...
Questions

Mathematics, 07.04.2020 09:14

Mathematics, 07.04.2020 09:14

Mathematics, 07.04.2020 09:15

English, 07.04.2020 09:16

Mathematics, 07.04.2020 09:16

Mathematics, 07.04.2020 09:16

Social Studies, 07.04.2020 09:16

History, 07.04.2020 09:16

Mathematics, 07.04.2020 09:17


Mathematics, 07.04.2020 09:18

History, 07.04.2020 09:18

Mathematics, 07.04.2020 09:19

Biology, 07.04.2020 09:19






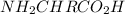 the ball and stick model gives an idea about the bonds arranged in this molecule. With the given information it is easy to identify that Carbon and Oxygen atoms in the double bond CO will have strongest partial positive charges.
the ball and stick model gives an idea about the bonds arranged in this molecule. With the given information it is easy to identify that Carbon and Oxygen atoms in the double bond CO will have strongest partial positive charges.

