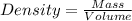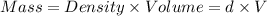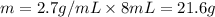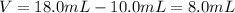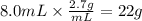Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1
You know the right answer?
Asample of aluminum is placed in a 25-ml graduated cylinder containing 10.0 ml of water. the level o...
Questions




Mathematics, 27.06.2020 03:01



Mathematics, 27.06.2020 03:01







Mathematics, 27.06.2020 03:01


Mathematics, 27.06.2020 03:01

Mathematics, 27.06.2020 03:01

Mathematics, 27.06.2020 03:01

Chemistry, 27.06.2020 03:01