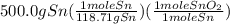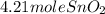Chemistry, 12.07.2019 13:30 juliannxkim
Sno2 + 2h2 → sn + 2h2o tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn? a. 1.57 b. 4.21 c. 634.8 d. 59,350

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
Sno2 + 2h2 → sn + 2h2o tin oxide reacts with hydrogen to produce tin and water. how many moles of sn...
Questions



English, 28.12.2021 14:00



Mathematics, 28.12.2021 14:00



English, 28.12.2021 14:00

SAT, 28.12.2021 14:00

Mathematics, 28.12.2021 14:00







English, 28.12.2021 14:00

Computers and Technology, 28.12.2021 14:00

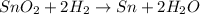
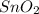 and Sn.
and Sn.