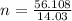Chemistry, 12.07.2019 21:00 tessalopezgarcia2345
The empirical formula of a chemical substance is ch2. the molar mass of a molecule of the substance is 56.108 g/mol. what is the molecular formula of the chemical substance? a: c2h4 b: c4h8 c: c3h4 d: c6h6

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
The empirical formula of a chemical substance is ch2. the molar mass of a molecule of the substance...
Questions





Mathematics, 11.12.2020 04:00

Mathematics, 11.12.2020 04:00

Mathematics, 11.12.2020 04:00

Law, 11.12.2020 04:00


Spanish, 11.12.2020 04:00


Mathematics, 11.12.2020 04:00



Mathematics, 11.12.2020 04:00





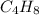 .
.