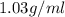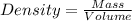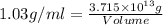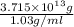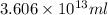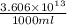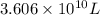Chemistry, 12.07.2019 22:00 jewelz5887
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals. it is obtained mostly from seawater, which contains about 1.30 g of mg for every kilogram of seawater. calculate the volume of seawater (in liters) needed to extract 5.33 × 104 tons of mg. seawater has a density of 1.03 g/ml. (1 ton = 2000 lb; 1 lb = 453.6 g) enter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals...
Questions


Physics, 21.05.2020 23:00





Computers and Technology, 21.05.2020 23:00

English, 21.05.2020 23:01


Mathematics, 21.05.2020 23:01


Physics, 21.05.2020 23:01


Mathematics, 21.05.2020 23:01


Advanced Placement (AP), 21.05.2020 23:01




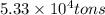 into gram.
into gram.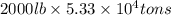
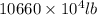 =
= 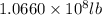
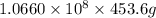
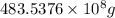 =
= 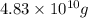
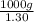 of seawater.
of seawater.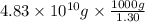 =
= 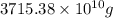
 of seawater.
of seawater.