

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
If the ka of a monoprotic weak acid is 5.4 × 10-6, what is the ph of a 0.14 m solution of this acid?...
Questions

Mathematics, 21.06.2021 17:50




Mathematics, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

Arts, 21.06.2021 17:50


Computers and Technology, 21.06.2021 17:50


Computers and Technology, 21.06.2021 17:50



History, 21.06.2021 17:50

English, 21.06.2021 17:50



Physics, 21.06.2021 17:50

History, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

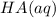 resembles the acid; as a weak acid (a small value of
resembles the acid; as a weak acid (a small value of 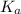 )
) 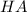 would partially dissociate to produce protons
would partially dissociate to produce protons  and
and 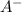 , its conjugate base. Let the final proton concentration (i.e.,
, its conjugate base. Let the final proton concentration (i.e., ![[H^{+}]](/tpl/images/0083/5038/cab36.png) ) be
) be  . (Apparently
. (Apparently 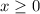 ) Construct the following RICE table:
) Construct the following RICE table: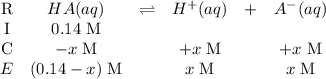
![\left\begin{array}{ccc}K_{a}&=&[H^{+}] \cdot [A^{-}] / [HA]\\&=&x^{2} /(0.14 - x)\end{array}\right](/tpl/images/0083/5038/ec898.png)
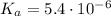
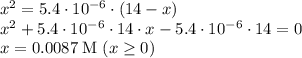
![\text{pH} = -\text{ln(}[H^{+}]\text{)} / \text{ln(}10\text{)} = 2.1](/tpl/images/0083/5038/74ad5.png)


