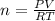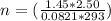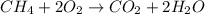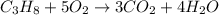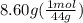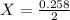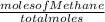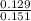A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 20°c. when this gas mixture is then burned in excess oxygen, 8.60 g of carbon dioxide is formed. (the other product is water.) what is the mole fraction of methane in the original gas mixture?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

You know the right answer?
A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 2...
Questions



Mathematics, 12.01.2020 05:31




History, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31



Mathematics, 12.01.2020 05:31

English, 12.01.2020 05:31