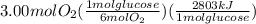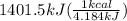Chemistry, 13.07.2019 08:00 zekrader18
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process releases 2803 kj per mole of glucose. when 3.00 mol of oxygen react in this way with glucose, what is the energy release in kcal? (hint: write a balanced equation for the combustion process.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process...
Questions




Mathematics, 30.07.2019 01:30

Biology, 30.07.2019 01:30



English, 30.07.2019 01:30


Mathematics, 30.07.2019 01:30




Mathematics, 30.07.2019 01:30

Business, 30.07.2019 01:30

Computers and Technology, 30.07.2019 01:30


Spanish, 30.07.2019 01:30

Social Studies, 30.07.2019 01:30

Mathematics, 30.07.2019 01:30