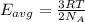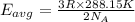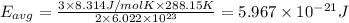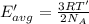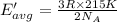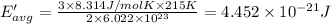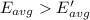How does a sample of helium at 15 °c compare to a sample of helium at 215 k? the helium at 15 °c has a higher average kinetic energy than the sample at 215 k. the helium at 15 °c has lower nuclear energy than the sample at 215 k. the helium at 15 °c has slower-moving atoms than the sample at 215 k. the helium at 15 °c has smaller atoms than the sample at 215 k.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
How does a sample of helium at 15 °c compare to a sample of helium at 215 k? the helium at 15 °c ha...
Questions

Physics, 21.01.2021 08:00

Biology, 21.01.2021 08:00




Social Studies, 21.01.2021 08:00

Geography, 21.01.2021 08:00

Mathematics, 21.01.2021 08:00


Mathematics, 21.01.2021 08:00



Mathematics, 21.01.2021 08:00


English, 21.01.2021 08:00

Mathematics, 21.01.2021 08:00

History, 21.01.2021 08:00

Medicine, 21.01.2021 08:00

Mathematics, 21.01.2021 08:00