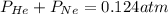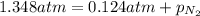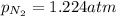Chemistry, 13.07.2019 09:30 shanicejordan
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 atm, what is the partial pressure of n2 in the mixture ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

You know the right answer?
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 at...
Questions




History, 07.04.2020 01:39




Mathematics, 07.04.2020 01:40

Chemistry, 07.04.2020 01:40


Mathematics, 07.04.2020 01:40


History, 07.04.2020 01:40

Mathematics, 07.04.2020 01:40



English, 07.04.2020 01:40

Mathematics, 07.04.2020 01:40


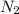 in the mixture is, 1.224 atm
in the mixture is, 1.224 atm
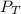 = total partial pressure of
= total partial pressure of 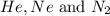 = 1.348 atm
= 1.348 atm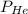 = partial pressure of helium
= partial pressure of helium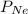 = partial pressure of neon
= partial pressure of neon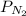 = partial pressure of nitrogen
= partial pressure of nitrogen