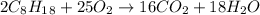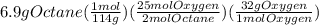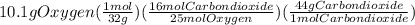Chemistry, 13.07.2019 09:30 cyaransteenberg
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 6.9 g of octane is mixed with 10.1 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon di...
Questions


English, 22.11.2021 14:00

History, 22.11.2021 14:00

Chemistry, 22.11.2021 14:00

Mathematics, 22.11.2021 14:00




Mathematics, 22.11.2021 14:00


Mathematics, 22.11.2021 14:00



Mathematics, 22.11.2021 14:00

Health, 22.11.2021 14:00

History, 22.11.2021 14:00

Mathematics, 22.11.2021 14:00