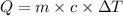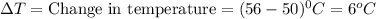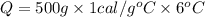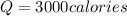Chemistry, 13.07.2019 11:30 lisapcarroll
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c to 56°c? the specific heat capacity of water is 1.00 cal/g°c. 83 calories 494 calories 506 calories 3,000 calories

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c...
Questions

History, 27.09.2019 19:30




Biology, 27.09.2019 19:30

Biology, 27.09.2019 19:30


History, 27.09.2019 19:30

Mathematics, 27.09.2019 19:30


Health, 27.09.2019 19:30

Computers and Technology, 27.09.2019 19:30





History, 27.09.2019 19:30

Mathematics, 27.09.2019 19:30

Physics, 27.09.2019 19:30

History, 27.09.2019 19:30