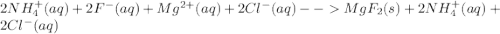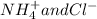Write the net ionic equation for this reaction occurring in water: ammonium fluoride and magnesium chloride are mixed to form magnesium fluoride and ammonium chloride. 1. no reaction occurs. 2. 2 nh+ 4 + 2 cl− → 2 nh4cl 3. 2 f− + mg2+ → mgf2 4. 2 nh4f + 2 cl− → 2 f− + 2 nh4cl 5. 2 f− + mgcl2 → mgf2 + 2 cl−

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Write the net ionic equation for this reaction occurring in water: ammonium fluoride and magnesium...
Questions


Mathematics, 07.10.2021 21:30


Computers and Technology, 07.10.2021 21:30


Engineering, 07.10.2021 21:30




Social Studies, 07.10.2021 21:30


Mathematics, 07.10.2021 21:30

Physics, 07.10.2021 21:30


English, 07.10.2021 21:30


Mathematics, 07.10.2021 21:30



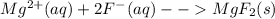

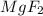 will not split into ions as it is insoluble in water.
will not split into ions as it is insoluble in water.