
Chemistry, 13.07.2019 12:30 codeyhatch142
One way to identify a substance is to compare the density of the substance with the density of a known substance. suppose a cube of a silver-colored metal with a volume of 64 cm3 has a mass of 672 g. the density of pure silver is known to be 10.5 g/cm3. based on this information, could the metal be pure silver? a. no. the density is greater than pure silver's. b. no. the density is less than pure silver's. c. yes. the density equals that of pure silver.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
One way to identify a substance is to compare the density of the substance with the density of a kn...
Questions




Mathematics, 09.12.2019 12:31



History, 09.12.2019 12:31

Mathematics, 09.12.2019 12:31




Geography, 09.12.2019 12:31




Mathematics, 09.12.2019 12:31


Business, 09.12.2019 12:31

Mathematics, 09.12.2019 12:31

History, 09.12.2019 12:31

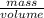 =
= 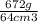 cm³
cm³

