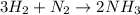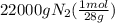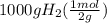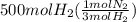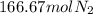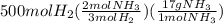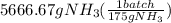Achemical company makes ammonia by reacting nitrogen with hydrogen. the company needs to make 70 batches of ammonia for a client. each batch contains 175 grams. they have 22,000 grams of n2 and 1000 grams of h2. will they be able to make enough ammonia to fill the order? (you may have to balance the equation.) h2 + n2 -> nh3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Achemical company makes ammonia by reacting nitrogen with hydrogen. the company needs to make 70 bat...
Questions


Geography, 28.10.2019 05:31


Arts, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31

Computers and Technology, 28.10.2019 05:31



Mathematics, 28.10.2019 05:31

Business, 28.10.2019 05:31

English, 28.10.2019 05:31

Biology, 28.10.2019 05:31

Chemistry, 28.10.2019 05:31




Mathematics, 28.10.2019 05:31


Mathematics, 28.10.2019 05:31