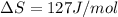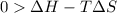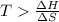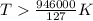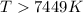Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3

You know the right answer?
At what temperature is the following reaction feasible: hcl(g) + nh3(g) -> nh4cl(s)?
Questions



Mathematics, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20



English, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20


Mathematics, 10.06.2021 19:20






Mathematics, 10.06.2021 19:20

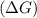 should be negative or
should be negative or 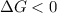 .
.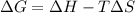
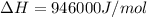 and
and