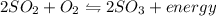Chemistry, 13.07.2019 18:00 Woodsydal2390
Which situation would cause the following equilibrium reaction to decrease the formation of the products? 2so2 (g) + o2 (g) two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2so3 (g) + energy increase the temperature decrease the volume increase the pressure decrease the temperature

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

You know the right answer?
Which situation would cause the following equilibrium reaction to decrease the formation of the prod...
Questions



English, 17.05.2021 16:40

Mathematics, 17.05.2021 16:40




Engineering, 17.05.2021 16:40



Mathematics, 17.05.2021 16:50

Arts, 17.05.2021 16:50


Social Studies, 17.05.2021 16:50





Social Studies, 17.05.2021 16:50