
Chemistry, 13.07.2019 18:30 adyenamaie02
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphorus and excess oxygen. in part b, you found the amount of product (3.40 mol p2o5 ) formed from the given amount of oxygen and excess phosphorus. now, determine how many moles of p2o5 are produced from the given amounts of phosphorus and oxygen. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphor...
Questions


Mathematics, 16.07.2020 14:01



Mathematics, 16.07.2020 14:01

Biology, 16.07.2020 14:01



Mathematics, 16.07.2020 14:01





Mathematics, 16.07.2020 14:01




Biology, 16.07.2020 14:01

Physics, 16.07.2020 14:01

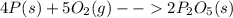
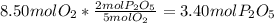
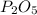 is formed from P (limiting reactant):
is formed from P (limiting reactant):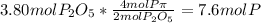
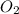 =
= 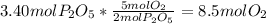
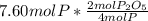
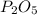 produced from P are
produced from P are 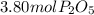
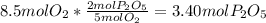
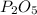 produced will be 3.40 mol
produced will be 3.40 mol

