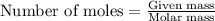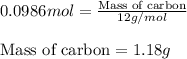Chemistry, 13.07.2019 18:30 naomicervero
C(s)+o2(> co2(g) how many grams of carbon should be burned in an excess of oxygen at stp to obtain 2.21 l of carbon dioxide? 1.18 g 2.21 g 4.12 g 4.34 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

You know the right answer?
C(s)+o2(> co2(g) how many grams of carbon should be burned in an excess of oxygen at stp to obtai...
Questions

Mathematics, 03.04.2020 04:11






Mathematics, 03.04.2020 04:11


Arts, 03.04.2020 04:11





Chemistry, 03.04.2020 04:11

History, 03.04.2020 04:11

Mathematics, 03.04.2020 04:11



Mathematics, 03.04.2020 04:11

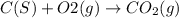
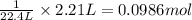 of carbon dioxide gas.
of carbon dioxide gas.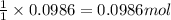 of carbon.
of carbon.