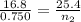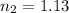Chemistry, 13.07.2019 20:00 lif3isanenigma
Aballoon that contains 0.750 moles of gas has a volume of 16.8 l. if the balloon expands to a volume of 25.4 l at a constant pressure and temperature, how many moles of gas would the balloon contain? 0.496 mol 0.882 mol 1.13 mol 6.45 mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Aballoon that contains 0.750 moles of gas has a volume of 16.8 l. if the balloon expands to a volume...
Questions

Social Studies, 11.07.2019 20:00

Mathematics, 11.07.2019 20:00



Mathematics, 11.07.2019 20:00


Spanish, 11.07.2019 20:00

Mathematics, 11.07.2019 20:00


Mathematics, 11.07.2019 20:00

Social Studies, 11.07.2019 20:00

Mathematics, 11.07.2019 20:00

Chemistry, 11.07.2019 20:00

Spanish, 11.07.2019 20:00


Mathematics, 11.07.2019 20:00

Advanced Placement (AP), 11.07.2019 20:00



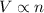 (At constant temperature and pressure)
(At constant temperature and pressure) 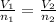
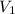 = initial volume of gas = 16.8 L
= initial volume of gas = 16.8 L
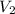 = final volume of gas = 25.4 L
= final volume of gas = 25.4 L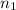 = initial number of moles = 0.750
= initial number of moles = 0.750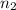 = final number of moles = ?
= final number of moles = ?