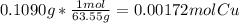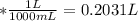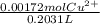Chemistry, 13.07.2019 20:00 masonorourke
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and then water is added to make a total volume of 203.1 ml. (calculate the molarity of cu2+.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

You know the right answer?
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and t...
Questions

Mathematics, 18.03.2021 01:10



Social Studies, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Chemistry, 18.03.2021 01:10





Mathematics, 18.03.2021 01:10

English, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10