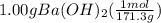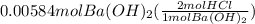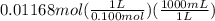Chemistry, 13.07.2019 21:30 ansuaprajita1506
Calculate the volume of 0.100 m hcl required to neutralize 1.00 g of ba(oh)2 (molar mass = 171.3 g/mol).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Calculate the volume of 0.100 m hcl required to neutralize 1.00 g of ba(oh)2 (molar mass = 171.3 g/m...
Questions


Mathematics, 28.04.2021 17:00


Mathematics, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00



Mathematics, 28.04.2021 17:00


Mathematics, 28.04.2021 17:00

Geography, 28.04.2021 17:00

Spanish, 28.04.2021 17:00


History, 28.04.2021 17:00

Physics, 28.04.2021 17:00

History, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00


History, 28.04.2021 17:00

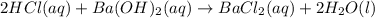
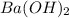 react in 2:1 mol ratio.
react in 2:1 mol ratio.