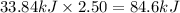Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: what is the enthalpy change when 2.50 mol of nitrogen dioxide decomposes? 13.5 kj of energy released 13.5 kj of energy absorbed 84.6 kj of energy released 84.6 kj of energy absorbed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: wh...
Questions

Physics, 12.03.2021 21:50







Social Studies, 12.03.2021 21:50

Chemistry, 12.03.2021 21:50

Mathematics, 12.03.2021 21:50

Biology, 12.03.2021 21:50


Mathematics, 12.03.2021 21:50


History, 12.03.2021 21:50


Mathematics, 12.03.2021 21:50

History, 12.03.2021 21:50

Mathematics, 12.03.2021 21:50

Mathematics, 12.03.2021 21:50

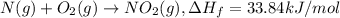
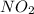 is formed = 33.84 kJ
is formed = 33.84 kJ