
Chemistry, 13.07.2019 22:30 lilyrockstarmag
Calculate the mass of oxygen in 350 g of al(c2h3o2)3 a 57 grams b 164.5 grams c 39.17 grams d 19.58 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Calculate the mass of oxygen in 350 g of al(c2h3o2)3 a 57 grams b 164.5 grams c 39.17 grams d 19.58...
Questions

Mathematics, 04.09.2020 19:01

Biology, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01



Mathematics, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01

Biology, 04.09.2020 19:01



Mathematics, 04.09.2020 19:01


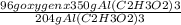 of oxygen
of oxygen

