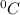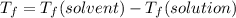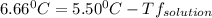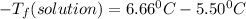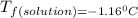Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Calculate the freezing point of a solution made from 22.0 g of octane (c8h18) dissolved in 148.0 g o...
Questions


Mathematics, 10.06.2021 04:40


Mathematics, 10.06.2021 04:40

Mathematics, 10.06.2021 04:40






Mathematics, 10.06.2021 04:40


Business, 10.06.2021 04:40

Mathematics, 10.06.2021 04:40

Mathematics, 10.06.2021 04:40


Mathematics, 10.06.2021 04:40



Computers and Technology, 10.06.2021 04:40

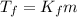
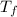 is the change in the freezing point of the solvent.
is the change in the freezing point of the solvent.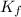 of benzene is 5.12
of benzene is 5.12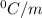
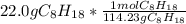 = 0.193 mol
= 0.193 mol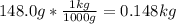
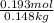
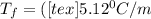 ) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />
) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />