
Chemistry, 14.07.2019 01:00 morgan3346
Acetylene gas, c2h2, burns in oxygen to produce carbon dioxide and water. if 60.5 g co2 are produced when 22.6 g c2h2 react with sufficient oxygen, what is the percent yield for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
Acetylene gas, c2h2, burns in oxygen to produce carbon dioxide and water. if 60.5 g co2 are produced...
Questions


Mathematics, 08.07.2021 15:40





Mathematics, 08.07.2021 15:40










Mathematics, 08.07.2021 15:40

Advanced Placement (AP), 08.07.2021 15:40

Mathematics, 08.07.2021 15:40

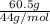
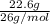
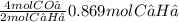
 x 100
x 100

