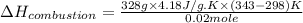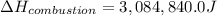Chemistry, 14.07.2019 02:00 autumperry7078
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the water rose from 298 k to 343 k. (the specific heat capacity of water is 4.18 j/k g.) what is the enthalpy of combustion? a. -61.7 kj b. 1,578.01 j c. 3,084,840.0 j d. 23,513,336 j

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2



Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the wate...
Questions

Mathematics, 25.12.2019 04:31



Mathematics, 25.12.2019 04:31

Mathematics, 25.12.2019 04:31


Biology, 25.12.2019 04:31

Mathematics, 25.12.2019 04:31






Mathematics, 25.12.2019 04:31

Mathematics, 25.12.2019 04:31

History, 25.12.2019 04:31


Geography, 25.12.2019 04:31


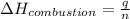
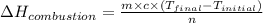
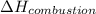 = enthalpy of combustion = ?
= enthalpy of combustion = ? = specific heat of water=
= specific heat of water= 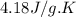
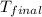 = final temperature = 343 K
= final temperature = 343 K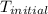 = initial temperature = 298 K
= initial temperature = 298 K