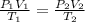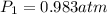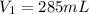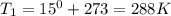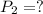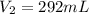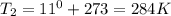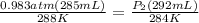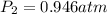Chemistry, 14.07.2019 02:30 ethanyayger
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next day, the same sample occupies 292 ml at a temperature of 11°c. what is the new pressure of the gas? 0.946 atm 1.00 atm 0.704 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

You know the right answer?
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next da...
Questions

History, 19.03.2021 21:30





Mathematics, 19.03.2021 21:30

Mathematics, 19.03.2021 21:30





Chemistry, 19.03.2021 21:30

English, 19.03.2021 21:30





Biology, 19.03.2021 21:30

Health, 19.03.2021 21:30