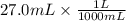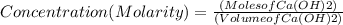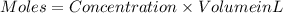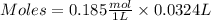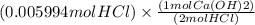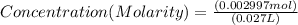Chemistry, 14.07.2019 02:30 fespinoza019
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what...
Questions


Mathematics, 14.01.2021 06:50

Mathematics, 14.01.2021 06:50

Mathematics, 14.01.2021 06:50

Mathematics, 14.01.2021 06:50




Mathematics, 14.01.2021 06:50

Advanced Placement (AP), 14.01.2021 06:50

English, 14.01.2021 06:50







Mathematics, 14.01.2021 07:00

Physics, 14.01.2021 07:00