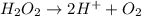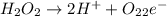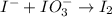Chemistry, 14.07.2019 02:30 madisonrparks
Question 8 how many electrons should be added to balance the following oxidation half-reaction, h2o2 imported asset 2 h+ + o2? none one two four question 9 what are the appropriate balanced half-reactions for the following reaction, which is carried out in an acidic solution: i− + io3− imported asset i2 ? i− imported asset i2 (oxidized) and io3 imported asset i2 (reduced) io3 imported asset i2 (oxidized) and i− imported asset i2 (reduced) 2 i− imported asset i2 + 2 e− (oxidized) and 2 io3− + 2 e− imported asset i2 (reduced) 2 i− imported asset i2 + 2 e− (oxidized) and 2 io3− + 10 e− imported asset i2 (reduced)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Question 8 how many electrons should be added to balance the following oxidation half-reaction, h2o2...
Questions




Computers and Technology, 27.05.2021 23:20











English, 27.05.2021 23:20




History, 27.05.2021 23:20

Mathematics, 27.05.2021 23:20

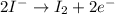 (oxidized) and
(oxidized) and 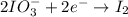 (reduced)
(reduced)